When maintaining aquatic life we are told to look out for pH and water hardness. That’s typically the end of fish store briefing, yet there is much more to learn about maintaining the perfect aquarium chemistry. Even where you live may have a huge impact on your water chemistry. By learning how to control these levels and the effects each parameter will have on your tank, owners everywhere can create a more suitable aquatic environment
pH: Acidic and Alkaline
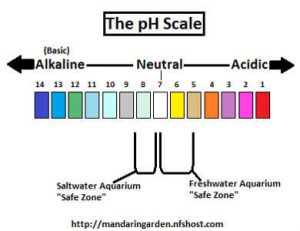
As the section states, pH determines how acidic or alkaline your water is. The level of pH is determined by the amount of hydrogen and hydroxide ions that are dissolved in a solution. The solution in this case being our water. pH ranges from 0 to 14, with 0 being the most acidic and 14 being highly alkaline. the middle number, 7, is considered neutral. As water’s pH moves from 7, each number value it moves makes the water 10 times more acidic or alkaline depending on if the pH rose or fell. For example a pH of 4 is 1000 times more acidic than a pH of 7, while a pH of 8 is 10 times more alkaline than 7. This is a result of the logarithmic scale pH follows.
Pure water is a neutral substance and changes to acid or alkaline when substances are added. These can be leeched out from rocks, fish waste, woods, chemicals such as “pH up” and even some substrates.
Freshwater aquariums will need to be kept between the pH range of 5.5 and 7.5, with a few exceptions such as chiclids who reach a pH of 8.4. Keeping the wrong pH for your fish, even if only slightly off, can be deadly for fish.
pH will affect your fishes ability to breathe, can damage skin, eyes and gills. More extreme effects can range from stress all the way to thickening of the gills, known as epithelial hyperplasia.
This is not to say your fishes personal condition will be the only negative affect unbalanced pH can bring. When the pH increases, ammonia becomes more toxic, making frequent water changes an extreme necessity. High alkalinity levels will see solids such as calcium precipitate out of the water, removing essential elements from the ecosystem. Even when the minerals are not needed, the high alkalinity will cause more degradation to occur on your aquariums equipment, lowering visibility into the tank and damaging filter or pumps.
Changing pH
While the easiest method to having the correct pH in the tank is to select fish matching your waters natural pH levels, many aquarists will want to keep fish out side of their normal waters levels. To house these fish we will alter the pH of our water, either raising or lowering the level until the fish can be kept safely.
Lowering pH:
For those finding their water too alkaline to house the fish they desire, we have several safe methods to lower the pH in the aquarium. Remember all pH changes must be done gradually. Sudden changes will endanger your fish.
- Adding woods to the tank will reduce your pH, but have the side affect of browning the water. By boiling the wood or soaking it multiple times before use owners can lessen color change. This browning affect does not harm your fish as the wood is simply releasing tannins.
- Adding Peat moss can create the same effect. To add the moss into your tank simply place it inside a filter bag or woman’s pantyhose. For increased effect keep the moss near high water flow areas.
- Processing water through a water softener or reverse osmosis. This can be done at start up or during water changes.
- Increasing CO2 levels will also serve to lower your pH naturally. Most aquarists don’t know this, but their plants will actually do this for them. that’s why for lower pH tanks we suggest creating a planted aquarium.
- Lastly you can purchase chemicals from your local stores to lower pH. While these will typically work they are only a temporary situation. Best used for emergency adjustments when you don’t have time for the other methods.
Raising pH:
When soft water causes aquarium troubles we seek to raise our pH levels. In saltwater tanks this is usually needed as they prefer a harder water in general. Even some freshwater fish such as chiclids prefer a higher pH.
- Adding calcareous rocks to your aquarium is the route most people will go without thought. Rocks serve as a natural decoration and can for beautiful aquascapes. Due to this practicality, adding rocks is our #1 suggestion.
- Crushed coral increases pH and can be used both as your aquariums substrate or placed inside of your filter. Many fish will enjoy the sand like composition of crushed coral.
- Adding baking soda will neutralize the acidic properties in your aquariums water, quickly raising your pH. Use with caution: Changing the pH too quickly can shock or even kill fish! The best way to use baking soda is during a water change. Adjust the pH of the new water before adding it into the tank, making sure to add the water slowly over an hour or two. A good amount to use is 3/4ths of a teaspoon for every 10 gallons of water.
- Once more many store products exist to raise pH. The effects are temporary and should be used as a last resort.
Water Hardness: kH & gH
Hardness is created mainly from carbonate, calcium and magnesium ions. Hard water is usually well buffered unlike soft water.
Water collects many dissolved substances when it is created through precipitation and during it’s stay in our tanks. As the water washes over rocks and other various metals the harder ions such as zinc, iron, calcium and magnesium dissolve into the water. Typically owners will only have calcium and magnesium with trace amounts of the other two metals.
When dealing with water hardness there are two types of hardness we need to address.
kH: alkalinity, which is often known as carbonate or temporary hardness.
gH: Aside from the temporary hardness lies permanent hardness. This is a measure of the ions in your water such as chlorides, sulfates and nitrates. Adding permanent and temporary hardness form the gH.
kH:
Temporary hardness measures the ability to absorb or buffer added acid without changing the pH. This will hold the pH in place, with a higher buffering capacity holding the pH even better. With this in mind owners should keep their kH high enough to prevent major changes to the systems pH between water changes.
If this sounds too good to be true, it can be. Water made hard with high kH that has a pH above your fishes level will be difficult to change. This makes the buffer more of a barrier in some cases.
Tanks on the opposite end with low kH will require much more attention to the pH, as they have little resistance to pH swings and rising nitrate levels.
As a note it is never recommended to use distilled water when making your tank. As the distilling process removes all kH, any changes in the water’s pH will be drastic.
Increasing kH:
- Increasing aeration in the environment will drive out carbon dioxide, increasing kH in an easy manner.
- Low amounts of baking soda, half a teaspoon to 15 gallons, can increase the kH without much change to the pH.
- Adding potassium carbonate will increase the kH of your water. It has the added effect of major nutritional value to plants in the aquarium.
- Store products, temporary solution etc etc.
Lowering kH:
- Adding carbon dioxide has always been a common solution to high kH. Inject slowly into your tank and measure the kH change if possible.
- Boiling water before adding it to the tank will help remove the ions, lowering both kH and gH. Allow water to cool before adding to your tank.
- Reverse osmosis water will remove most metals and ions, creating a purified, low kH environment.
- Store products here too.
gH:
This is general hardness, the dissolved concentration of ions in the water combined with your waters kH. The main ions in your tank will be calcium and magnesium and are what we will be focused on.
Housing fish with the incorrect gH will affect the transfer of nutrients, egg production and fertility, and internal organs of your fish. Typically gH will come in acceptable ranges, but old tank syndrome and rare water problems will cause owners major problems.
When measuring your gH with testing kits the levels will be listed here:
| dH | ppm | gH |
| 0-8 dH | 0-140 ppm | Soft |
| 8-12 dH | 140-210 ppm | Medium |
| 12-30 dH | 210-530 ppm | Hard |
Units:
dH, degrees hardness, is the molar concentration of calcium carbonate, where 1 dH is 10 mg of calcium oxide per liter. ppm is the parts per million of calcium carbonate. Depending on your testing equipment your results will be in one of the two units.
Changing gH:
Because this is the general hardness the same steps should be taken to both raise and lower gH as adjusting the overall pH.
Rounding it Out
Maintaining the right pH is a vital step in aquarium care. Be sure to make all changes gradually and monitor levels. Once the desired pH has been achieved testing can be done less frequently, depending on the kH levels. Ensure there will be no pH changes when adding your decorations and if there will be, take these into account when creating your original pH level. If your levels are amiss frequent water changes can help to level the pH out while you get the problem under control.